با سلام
مطالب مربوط به خورگی
 Welcome to Corrosion
Welcome to Corrosion 


Corrosion Theory
Historical Theories on Corrosion
FACTORS INFLUENCING CORROSION REACTIONS
مطالب مربوط به خورگی


Humans have most likely been trying to understand and control corrosion for as long as they have been using metal objects. The most important periods of prerecorded history are named for the metals that were used for tools and weapons (Iron Age, Bronze Age). With a few exceptions, metals are unstable in ordinary aqueous environments. Metals are usually extracted from ores through the application of a considerable amount of energy.
Certain environments offer opportunities for these metals to combine chemically with elements to form compounds and return to their lower energy levels. A modern and comprehensive document on the subject is the second edition of the classic CORROSION BASICS textbook. Some excerpts of that document are used here.
Corrosion is the primary means by which metals deteriorate. Most metals corrode on contact with water (and moisture in the air), acids, bases, salts, oils, aggressive metal polishes, and other solid and liquid chemicals. Metals will also corrode when exposed to gaseous materials like acid vapors, formaldehyde gas, ammonia gas, and sulfur containing gases. Corrosion specifically refers to any process involving the deterioration or degradation of metal components. The best known case is that of the rusting of steel. Corrosion processes are usually electrochemical in nature, having the essential features of a battery.
When metal atoms are exposed to an environment containing water molecules they can give up electrons, becoming themselves positively charged ions, provided an electrical circuit can be completed. This effect can be concentrated locally to form a pit or, sometimes a crack, or it can extend across a wide area to produce general wastage. Localized corrosion that leads to pitting may provide sites for fatigue initiation and, additionally, corrosive agents like seawater may lead to greatly enhanced growth of the fatigue crack. Pitting corrosion also occurs much faster in areas where microstructural changes have occurred due to welding operations.
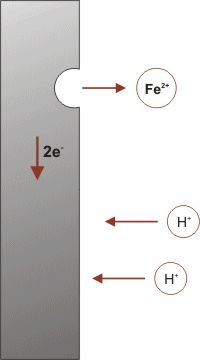 The corrosion process (anodic reaction) of the metal dissolving as ions generates some electrons, as shown in the simple model on the left, that are consumed by a secondary process (cathodic reaction). These two processes have to balance their charges. The sites hosting these two processes can be located close to each other on the metal's surface, or far apart depending on the circumstances. This simple observation has a major impact in many aspects of corrosion prevention and control, for designing new corrosion monitoring techniques to avoiding the most insidious or localized forms of corrosion.
The corrosion process (anodic reaction) of the metal dissolving as ions generates some electrons, as shown in the simple model on the left, that are consumed by a secondary process (cathodic reaction). These two processes have to balance their charges. The sites hosting these two processes can be located close to each other on the metal's surface, or far apart depending on the circumstances. This simple observation has a major impact in many aspects of corrosion prevention and control, for designing new corrosion monitoring techniques to avoiding the most insidious or localized forms of corrosion.
Mixed potential theory, Other corrosion models, Why Metals Corrode?
Evolution of Hydrogen on Zinc: a Model and a PictureCertain environments offer opportunities for these metals to combine chemically with elements to form compounds and return to their lower energy levels. A modern and comprehensive document on the subject is the second edition of the classic CORROSION BASICS textbook. Some excerpts of that document are used here.
Corrosion is the primary means by which metals deteriorate. Most metals corrode on contact with water (and moisture in the air), acids, bases, salts, oils, aggressive metal polishes, and other solid and liquid chemicals. Metals will also corrode when exposed to gaseous materials like acid vapors, formaldehyde gas, ammonia gas, and sulfur containing gases. Corrosion specifically refers to any process involving the deterioration or degradation of metal components. The best known case is that of the rusting of steel. Corrosion processes are usually electrochemical in nature, having the essential features of a battery.
When metal atoms are exposed to an environment containing water molecules they can give up electrons, becoming themselves positively charged ions, provided an electrical circuit can be completed. This effect can be concentrated locally to form a pit or, sometimes a crack, or it can extend across a wide area to produce general wastage. Localized corrosion that leads to pitting may provide sites for fatigue initiation and, additionally, corrosive agents like seawater may lead to greatly enhanced growth of the fatigue crack. Pitting corrosion also occurs much faster in areas where microstructural changes have occurred due to welding operations.

Mixed potential theory, Other corrosion models, Why Metals Corrode?
Historical Theories on Corrosion
FACTORS INFLUENCING CORROSION REACTIONS
The following is an excerpt form a textbook that was published more that eighty years ago by Frank N. Speller. If you read this text carefully and compare it with what is said in modern corrosion basic courses, you may be surprised on how little the messages have changed. (see FACTS)
In any discussion of the mechanism of a chemical reaction it is advisable to separate the factors which determine the tendency or driving force of the reaction to proceed from those which influence the rate of the reaction made possible by the existence of this tendency. This tendency is an expression of the fact that the system is not in a state of equilibrium (or inherent stability); it is measured by the difference in energy between the initial and final state of the system for any particular case. In most cases the observed rate is determined not by the absolute magnitude of this tendency but by other factors, which depend primarily upon the environment.
In considering the group of three typical reactions involved in corrosion, we shall denote as primary factors those which determine the tendency of the metal to corrode and thus influence its initial rate of solution and as secondary factors those which influence the rate of the subsequent reactions. This term in no wise implies that these secondary factors are of lesser importance; in fact, by influencing the nature and distribution of the final corrosion products, they usually determine the ultimate rate of corrosion, and the useful life of the metal, in each environment.
In the general case, some one or two of the many factors involved exert outstanding influence upon the ultimate rate of corrosion; these we term controlling or dominant factors. In general, the primary factors have to do with the metal (or alloy) itself ; the secondary factors more with the specific environment. It is convenient to divide them in this way, although no sharp distinction can be made.
Accordingly on this basis we list below some of the more important factors, discussing their general significance with respect to the mechanism of corrosion, and postponing until later chapters the detailed discussion of others.
Factors Associated Mainly with the Metal
In considering the group of three typical reactions involved in corrosion, we shall denote as primary factors those which determine the tendency of the metal to corrode and thus influence its initial rate of solution and as secondary factors those which influence the rate of the subsequent reactions. This term in no wise implies that these secondary factors are of lesser importance; in fact, by influencing the nature and distribution of the final corrosion products, they usually determine the ultimate rate of corrosion, and the useful life of the metal, in each environment.
In the general case, some one or two of the many factors involved exert outstanding influence upon the ultimate rate of corrosion; these we term controlling or dominant factors. In general, the primary factors have to do with the metal (or alloy) itself ; the secondary factors more with the specific environment. It is convenient to divide them in this way, although no sharp distinction can be made.
Accordingly on this basis we list below some of the more important factors, discussing their general significance with respect to the mechanism of corrosion, and postponing until later chapters the detailed discussion of others.
Factors Associated Mainly with the Metal
- Effective electrode potential of a metal in a solution
- Overvoltage of hydrogen on the metal
- Chemical and physical homogeneity of the metal surface
- Inherent ability to form an insoluble protective film
Factors Which Vary Mainly with the Environment
- Hydrogen-ion concentration (pH) in the solution
- Influence of oxygen in solution adjacent to the metal
- Specific nature and concentration of other ions in solution
- Rate of flow of the solution in contact with the metal
- Ability of environment to form a protective deposit on the metal
- Temperature
- Cyclic stress (corrosion fatigue)
- Contact between dissimilar metals or other materials as affecting localized corrosion.

