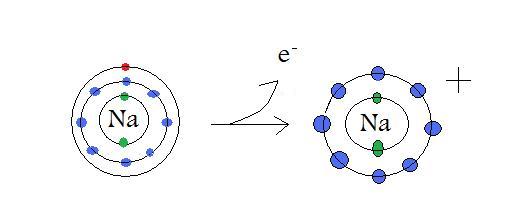
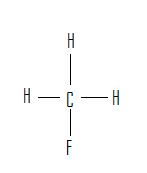
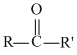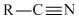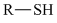تقارن در شيمي زمينه حل بسياري از مسائل علم شيمي مانند: ساختار ملكول.ساختار الكترون.
تشكيل پيوند و هيبريداسيون همچنين پيش بيني طيف زير قرمز را فراهم ميكند.
در اينجا به بررسي تقارن ملكولهاي مجزا ميپردازيم كه تقارن آنها به تقارن نقطه اي موسوم است.
مفاهيم مهم در تقارن
1-عنصر تقارن: واقعيتي هندسي مانند خط صفحه و يا نقطه كه يك يا چند عمل تقارن نسبت به
آن انجام ميشود.
2-عمل تقارن: حركت يا عملي كه انجام آن روي ملكول يا سيستم وضعيت جديدي ايجاد كند طوري
كه وضعيت جديد از وضعيت قبلي ملكول يا سيستم غير قابل تمييز باشد.
پس وضعيت جديد شبيه به وضعيت قبليست اما معادل با آن نيست.
در تقارن اين دو مفهوم به نحو جدايي ناپذيري به هم مربوط اند.چون عمل تقارني فقط به پشتوانه يك
عنصر تقارن انجام ميشود و به همين ترتيب وجود عنصر تقارن تنها زماني براي سيستم اهميت دارد
كه بتوان روي آن يك عمل تقارني انجام داد.
5 نوع عنصر تقارن و عمل تقارني براي مشخص كردن تقارن ملكولي:
1- عنصر يكسانيE
2-محور چرخشي Cn
3-مركز وارونگي يا مركز تقارن i
4-صفحه تقارن يا صفحه آيينه اي σ
5-محور چرخشي-انعكاسي Sn
1- عنصر يكساني E
عمل يكساني هيچ تغييري در ملكول ايجاد نميكند و شامل چرخش ملكول به اندازه360درجه است.
پس هر شيء يا سيستمي داراي عمل يكساني E است.
این عمل ، هیچ تغییری در مولکول ایجاد نمیکند. هر مولکولی ، یک عمل یکسانی دارد، حتی اگر
هیچ تقارنی نداشته باشد.
نتيجه عمل يكساني روي نقطه اي به مختصات x y z را به صورت زير نشان ميدهند.
x1.y1.z1---------->x1.y1.z1
2- محور چرخشي
محور چرخشي متعارف به صورت Cnنشان داده ميشود و nمرتبه محور دوران است كه محوري است
فرضي و عمل تقارني چرخش به اندازهn/360 حول آن محور بطوريكه ملكول به حالتي مشابه حالت
اوليه اش باز گردد را موجب ميشود.
عمل چرخشی که چرخش متعارف نیز نامیده میشود، مستلزم چرخش به اندازه 360 بر n درجه
حول محور چرخش است.
به طور مثال C2 و C3 و C4 به ترتيب نشان دهنده دوران به اندازه 180 و 120 و 90 درجه است.
C1:
محور چرخشي C1 ملكول و اتم ها را به اندازه 360 درجه ميچرخاند پس معادل با عمل E ميباشد.
پس هر سيستمي داراي محور C1 است حتي محل قرار گرفتن آن مهم نيست چرا كه اين محور
را هر جادر نظر بگيريم و سيستم را نسبت به آن و با زاويه 360 درجه بچرخانيم به حالت اوليه باز
ميگردد.
C2:
براي استفاده از محور c2 بايد اتم مركزي حداقل دو ليگند داشته باشد تا با زاويه180درجه بچرخند.
مثال- ملكول آب را در نظر بگيريد.اين ملكول كاملا در صفحه دو بعدي قرار دارد پس محور آن هم در
همان صفحه تعريف خواهد شد.
با چرخش سيستم تحت c2 سيستم وضعيتي مشابه وضعيت قبل پيدا كرده اما با آن معادل نيست
چراكه مشاهده ميشود كه جاي اتم h1 و h2 باهم عوض شده.
توان گذاشته شده براي c تعداد دفعات اعمال c2 روي سيستم را مشخص ميكند.بنابراين توان دو
به اين معنيست كه چرخش سيستم به اندازه 180 درجه تحتc2 دو بار انجام شده كه سيستم را
به حالت اوليه بازميگرداند.
اگر محور فرضي C2 منطبق بر محور Z باشد هر نقطه اي به مختصاتx y z به صورت زير تغيير ميكند:
XYZ------->-X -Y Z
و اگر اين محور منطبق بر محور X باشد آنگاه خواهيم داشت:
X Y Z-------->X -Y -Z
CHCl[SUB]3[/SUB] نمونه ای از مولکولهایی است که دارای محور درجه سه (C[SUB]3[/SUB]) میباشند و در آن ، محور چرخش
بر محور پیوند C-H منطبق است. اگر چرخش C[SUB]3[/SUB] دوبار پشت سرهم انجام میگیرد، یک چرخش جدید
˚240حاصل میشود که با C[SUP]2[/SUP][SUB]3[/SUB] نشان داده شده و جزو اعمال تنقارن مولکولی نیز میباشد.
سه عمل متوالی C[SUB]3[/SUB] برابر عمل یکسانی است (C[SUP]3[/SUP][SUB]3[/SUB]=E) که در همه مولکولها وجود دارد.
بسیاری از مولکولها و اجسام دیگر محورهای چرخش متعددی دارند.
ملكول BF3 را در نظر بگيريد.
داراي محور چرخشي مرتبه 3 ميباشد.در اين ملكول محور تقارنC3 عمود بر صفحه كاغذ است.اين
محور از اتم بور عبور ميكند و باعث چرخش ملكول به اندازه120درجه در جهت حركت عقربه هاي
ساعت ميشود.
ساختار اول مشابه ساختارهاي دوم و سوم است و معادل با ساختار آخر.
بنابر اين ميتوان نتيجه گرفت هر Cn به توان n و به عبارت ديگر هرمحور چرخشي با مرتبه n اگر n
مرتبه روي سيستم اعمال شود عملي معادل عنصر يكساني E دارد.
در مثال هاي قبل اين واقعيت مشهود است.
براي مشخص كردن جهت محورهاي مختصات محوري كه بالا ترين مرتبه را دارد محور اصلي منطبق
بر محور Z در نظر گرفته ميشود و بيشترين درجه چرخش
علاوه بر محور هاي مرتبه 2 و مرتبه 3 ملكول هاي شناخته شده اي نيز وجود دارند كه از محور هاي
بالاتري برخوردارند و تا مرتبه 8 ادامه دارند.
دانههای برف ، نمونههایی در این مورد هستند، با شکلهای پیچیده ای که معمولا شش گوشهای
تقریبا مسطح است. خطی که عمود بر صفحه دانه برف از مرکز آن گذشته ، دربرگیرنده یک محور درجه
دو (C[SUB]2[/SUB]) ، یک محور درجه سه (C[SUB]3[/SUB]) و یک محور درجه شش (C[SUB]6[/SUB]) است. دقت کنید که چرخش به اندازه
˚240 (C[SUP]2[/SUP][SUB]3[/SUB]) و ˚300 (C[SUP]5[/SUP][SUB]6[/SUB]) نیز جزو اعمال تقارن دانه برف میباشند.
همچنین دو مجموعه سهتایی دیگر از محورهای C[SUB]2[/SUB] در صفحه دانه برف وجود دارد که یک مجموعه از نقطه
های متقابل و مجموعه دیگر از وسط اضلاع میان نقطهها میگذرد. در مولکولهای دارای بیش از یک محور
چرخشی ، محور C[SUB]n[/SUB] دارای بزرگترین مقدار n ممکن بهعنوان محور چرخش با بزرگترین مرتبه یا محور اصلی
تعیین میشود.
محور چرخش با بزرگترین مرتبه در دانه برف ، محور C[SUB]6[/SUB] است. (در موقع انتقال به مشخصات کارتزین ، محور
C[SUB]n[/SUB] با بزرگترین مرتبه معمولا بهعنوان محور Z انتخاب میشود). در صورت لزوم ، محورها C[SUB]2[/SUB] عمود بر محور
اصلی را با پریم مشخص میکنند. یک تک پریم نشان میدهد که محور از داخل چندین اتم مولکول میگذرد،
در حالیکه یک جفت پریم نشان میدهد که محور از بین اتمها میگذرد.
3-مركز وارونگي يا مركز تقارن i
این عمل ، کمی پیچیدهتر است. هر نقطه از وسط مرکز مولکول به موقعیتی مقابل موقعیت اولیه حرکت
میکند، بهطوریکه فاصله اش از نقطه مرکزی برابر با فاصله ای باشد که در آغاز داشت.
اتان در حالت صورتبندی نامتقابل نمونه ای از مولکولهایی است که دارای مرکز وارونگی میباشند. بسیاری
از مولکولها که در نگاه اول به نظر میرسد مرکز وارونگی دارند، فاقد آن هستند. متان و مولکولهای چهار
وجهی دیگر ، نمونههایی از این مولکولها هستند.
اگر دو اتم هیدروژن یک مدل متان در صفحه عمودی سمت راست و دو اتم هیدروژن دیگرش در صفحه افقی
در چپ نگاه داشته شود، عمل وارونگی دو هیدروژن واقع در صفحه افقی را به سمت راست و دو هیدروژن
واقع در صفحه عمودی را به سمت چپ منتقل میکند. پس متان ، عمل وارونگی ندارد، چون جهتگیری
مولکول پس از عمل i با جهتگیری اولیه متفاوت است.
بطور کلی، چهار وجهیها، مسطح مثلثیها، پنج ضلعیها مرکز وارونگی ندارند. مربعها، متوازیالاضلاعها،
اجسام راستگوشه و دانههای برف مرکز وارونگی دارند.
4-صفحه تقارن يا صفحه آيينه اي σ
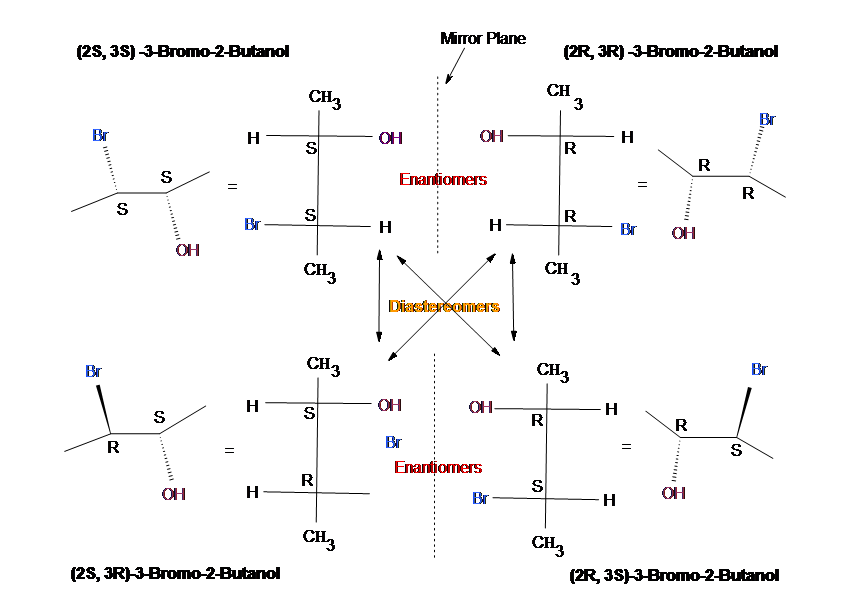
عمل انعکاسی (σ) ، موقعی وجود دارد که مولکول ، دارای یک صفحه آینهای باشد. اگر جزئیاتی نظیر آرایش
موها و محل اندامهای داخلی را در نظر نگیریم، بدن انسان دارای یک صفحه آینهای چپ – راست میباشد.
بسیاری از مولکولها، صفحه آینهای دارند، اگرچه ممکن است در نگاه اول آشکار نباشد.
عمل انعکاس، جای چپ و راست را عوض میکند، مانند اینکه هر نقطه بطور عمود از میان صفحه به موقعیتی
دقیقا در همان فاصله از صفحه که در آغاز بود، حرکت کرده است.
مولکولها میتوانند به هر تعدادی صفحه آینهای داشته باشند.
اجسام خطی نظیر یک مداد چوبی گرد یا مولکولهای همچون استیلن و کربن دیاکسید دارای تعداد نامحدودی
صفحه آینهای هستند که همه آنها دربرگیرنده محور مرکزی جسم میباشند.
5-محور چرخشي-انعكاسي Sn
این عمل که گاهی اوقات ، چرخش نامتقارن نامیده میشود، مستلزم چرخش به اندازه 360 بر n درجه و به
دنبال آن ، انعکاس از صفحه عمود بر محور چرخش میباشد. برای مثال ، در متان ، خطی که از وسط کربن
عبور کرده و زاویه میان هیدروژنها را در طرفین نصف میکند، یک محور S[SUB]4[/SUB] میباشد. از این نوع خط سه تا و
در کل سه محور S[SUB]4[/SUB] وجود دارد.
این عمل ، مستلزم چرخش مولکول به اندازه ˚90 و سپس انعکاس از صفحه آینهای عمود میباشد. دو عمل
متوالی S[SUB]n[/SUB] یک محور C[SUB]n[/SUB]/2 ایجاد میکند. در متان ، دو عمل S[SUB]4[/SUB] یک C[SUB]2[/SUB] ایجاد میکند.
بعضی وقتها ممکن است محور S[SUB]n[/SUB] مولکول با محور C[SUB]n[/SUB] آن منطبق باشد. مثلا دانههای برف ، علاوه بر محورهای
چرخش اشاره شده در بالا ، محورهای S[SUB]2[/SUB] ، S[SUB]3[/SUB] و S[SUB]6[/SUB] منطبق بر محور C[SUB]6[/SUB] نیز دارند.
دقت کنید که محور S[SUB]2[/SUB] با وارونگی و محور S[SUB]1[/SUB] با صفحه آینهای یکسانی هستند. در مورد اول ، نماد i و در مورد
دوم نماد σ ترجیح داده میشود.